Home Health Lab Startup Bisu Collaborates With Polaris Dawn to Develop New Ways to Monitor Astronaut Health
 Bisu’s “lab-on-a-chip” urine and saliva analyzer is intended for both consumer and astronaut use.
Bisu’s “lab-on-a-chip” urine and saliva analyzer is intended for both consumer and astronaut use.
TOKYO – December 1, 2022 – (Newswire.com)
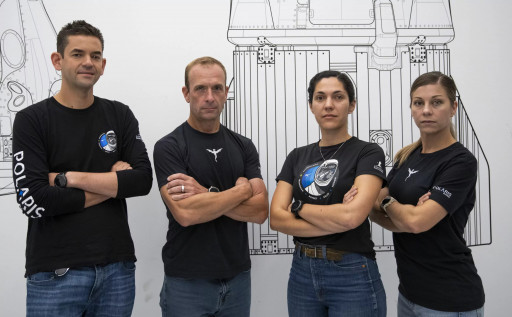
Traveling in space leads to bone loss, muscle loss, and an increased risk of kidney stone formation, often starting within the first 24 hours of spaceflight as calcium levels in bone and tissue decrease and are expelled in urine. A new research experiment, selected for inclusion in the upcoming Polaris Dawn spaceflight mission, aims to show if monitoring the first urine sample in the morning could provide a simple way to track astronaut health in space. If successful, the research will provide a pathway to use compact, new inflight monitoring techniques. The day’s first urine sample typically has the highest urine calcium levels in the day and taking measurements from this sample might offer a workable way to assess in-flight bone loss and kidney stone risk. As both space accessibility and long-duration human spaceflights increase, keeping crews healthy by providing personalized, targeted countermeasures will be essential. This will require small, easy-to-use, low-power devices that can provide actionable information using easily obtainable samples of urine or saliva, such as a first-morning void.
Bisu is a startup that helps people improve their health and fitness through lab-grade testing at home. Working with former astronaut Jay Buckey, MD, Professor of Medicine and Director of the Space Medicine Innovations Lab at the Geisel School of Medicine at Dartmouth, Bisu will support the post-flight analysis of first-morning void urine samples gathered from the Polaris Dawn crew to assess the effect of spaceflight on markers of bone loss, muscle loss, and kidney stone risk. If successful, this research could pave the way for inflight monitoring with new technologies, such as the microfluidic urine and saliva analyzer Bisu has developed. Because weightlessness affects people differently, this kind of in-flight monitoring could provide targeted countermeasures.
“People will be spending longer times in space, and they may not be able to do the extensive countermeasure programs currently used on the space station,” says Prof. Jay Buckey. “We need simple ways to monitor people while they are in space so that the countermeasure program can be targeted to each person’s individual needs. This research puts us on the path toward that.”
“We’re delighted to be working with Polaris Dawn and Prof. Buckey on this pioneering research,” says Daniel Maggs, co-founder & CEO at Bisu. “This research reflects Bisu’s commitment to advancing human health by making valuable health data accessible to all – whether on Earth or in space.”
About Polaris Dawn
Polaris Dawn is the first of the Polaris Program’s three human spaceflight missions. SpaceX is targeting no earlier than March 2023 for Falcon 9’s launch of Dragon and the Polaris Dawn crew from Launch Complex 39A at NASA’s Kennedy Space Center in Florida. Polaris Dawn endeavors to reach the highest Earth orbit ever flown, attempt the first-ever commercial spacewalk, conduct extensive research to further our understanding of human health on Earth and during future long-duration spaceflights, and test Starlink’s laser-based communications in space. For more information, visit polarisprogram.com/dawn.
About Bisu
Bisu is a healthtech startup that provides personalized, preventative advice at home, through nutrition and hormone tests using microfluidic “lab-on-a-chip” technology. In late 2021, Bisu announced a $3.2m seed round and collaboration with ASICS, took home the Good Design Award in the wellness category, and won the US Sports & Fitness Industry Association’s startup challenge.
Bisu’s flagship product, Bisu Body Coach, is currently in beta and can be applied for at www.bisu.com.
About the Space Medicine Innovations Laboratory at the Geisel School of Medicine at Dartmouth
The Space Medicine Innovations Laboratory is devoted to solving biomedical problems presented by long-duration space flight. The lab has performed work on bone loss, decompression sickness, motion sickness, and psychological countermeasures for isolation and confinement. The lab also advances work that began with NASA into other areas of research.
Please address all press inquiries to [email protected].
Contact Information:
Daniel Maggs
Press Release Service
by
Newswire.com
Original Source:
Home Health Lab Startup Bisu Collaborates With Polaris Dawn to Develop New Ways to Monitor Astronaut Health
Wedding Industry Icon Behind Catan Fashions Partners With Evergreen for Bridal Podcast
 Tales from Behind the Veil Tales from Behind the Veil with Patrice Catan
Tales from Behind the Veil Tales from Behind the Veil with Patrice Catan
CLEVELAND – December 1, 2022 – (Newswire….
Zeto Implements encevis’ Seizure Detection and Trending Algorithms
 The only zero-prep, full-montage, rapid EEG solution for clinical use now offers near real-time notification of ongoing seizures by using reliable FDA cleared seizure detection software
The only zero-prep, full-montage, rapid EEG solution for clinical use now offers near real-time notification of ongoing seizures by using reliable FDA cleared seizure detection software

Zeto-encevis PR
Zeto Implements encevis’ Seizure Detection and Trending Algorithms
SANTA CLARA, Calif. – December 1, 2022 – (Newswire.com)
Zeto, Inc., an innovative EEG brain monitoring company, announced today the integration of FDA cleared seizure detection and trending algorithms developed by encevis, now available as part of the Zeto EEG platform. encevis is powered by the AIT Austrian Institute of Technology, a renowned research and technology organization in Europe, and their EEG analysis software is widely utilized and peer reviewed by physicians across the world.
These new tools will help Zeto’s customers speed up EEG reviews. The trending module graphically reports several hours of EEG into a simple display to allow rapid assessment of brain states. The seizure detection component offers high sensitivity with low false alarm rates, matching and in parts surpassing industry benchmarks. Proven by three independent clinical publications, and harvesting the power of AI and deep learning, the software provides a detailed overview of seizures and suspicious EEG activity.
“According to current studies, 18% of critically ill patients with serious brain diseases suffer from unrecognized non-convulsive epileptic seizures, frequently leading to neurological disabilities. The joint efforts of Zeto and encevis will make EEG faster to read by automatically detecting and orienting the reader to abnormalities. Seizures will be automatically detected and marked in the EEG for review. Near real-time detection notifies medical staff about patients with ongoing clinical seizures,” said Dr. Tilmann Kluge, head of Medical Signal Analysis of AIT.
“We are excited to integrate encevis’ seizure detection and trending tools into our platform. Collaborative projects such as this ensure a seamless user experience and enable healthcare providers to offer the best care to their patients at an affordable price,” said Florian Strelzyk, Chief Sales Officer at Zeto.
Zeto EEG analysis features will continue to evolve as more third party integrations become available. Zeto is committed to driving innovation in brain monitoring by opening its platform to strategic projects and partnerships that empower health care providers to diagnose their patients rapidly and more effectively.
About Zeto
Zeto, Inc. is an award-winning, privately held medical technology company located in Santa Clara, California, that is focused on transforming the way electroencephalography (EEG) is performed at hospitals and clinics. Zeto’s revolutionary FDA-cleared EEG headset and cloud platform bring the traditional EEG procedure to the 21st century.
To learn more about Zeto’s products, including the remote EEG monitoring, please visit: https://zeto-inc.com or email us at [email protected].
About encevis
encevis is a part of the AIT Austrian Institute of Technology, Austria’s largest non-university research institute, is among the European research institutes a specialist in the key infrastructure issues of the future.
Learn more: https://www.encevis.com
Contact Information:
Irina Nazarova
Marketing Manager
9174351226
Press Release Service
by
Newswire.com
Original Source:
Zeto Implements encevis’ Seizure Detection and Trending Algorithms
Kelly Mahler, OTD, OTR/L, Releases New Big Book of Interoception Games
 The Big Book of Interoception Games
The Big Book of Interoception Games
LANCASTER, Pa. – December 1, 2022 – (Newswire.com)
…
Rising Obesity Rates; NextMed to the Rescue
 NextMed NextMed Weight Loss Program
NextMed NextMed Weight Loss Program
NEW YORK – December 1, 2022 – (Newswire.com)
…
AI-Fueled Endoscopy Capture and Clinical Trials Platform, Virgo Surgical Video Solutions, Receives Support From American Gastroenterological Association Venture Fund
 Virgo is first investment for new GI fund.
Virgo is first investment for new GI fund.
CARLSBAD, Calif. – December 1, 2022 …
SPOT Imaging Announces Cost-Saving Sample Tracking, Storage and Management Solutions
 New suite of time-saving products address pathology, cytology and histology laboratory staffing shortages and storage inefficiencies.
New suite of time-saving products address pathology, cytology and histology laboratory staffing shortages and storage inefficiencies.
SPOT Imaging:…
City of North Las Vegas Mayor Makes Nevada History
 Goynes-Brown Breaks Glass Ceiling as First Black Mayor in the State
Goynes-Brown Breaks Glass Ceiling as First Black Mayor in the State
City of North Las Vegas Mayor Pamela Goynes-Brown Makes Nevada History New No…
Ilkin Rustemzadeh’s New Book ‘Tale of Darkness’ is a Personal Account of the Author’s Time Imprisoned Under False Charges After Being Targeted for Political Activism

NEW YORK – December 1, 2022 – (Newswire.com)
Fulton Books author Ilkin Rustemzadeh, a political activist and native of Azerbaijan, has completed his most recent book “Tale of Darkness”: a gripping and poignant memoir of the author’s time in prison and the political circumstances that led to his false arrest and detainment.
Author Ilkin Rustemzadeh writes, “Azerbaijan—this is a little country known for energy resources and human rights violations in the border of Europe and Asia. But it is in a very important geopolitical position. For this reason, in most cases, the democratic world remains silent on human rights abuses. Well, what are the realities of this country? Second North Korea or last secular Muslim country with beautiful buildings and great lights? A witness of what happens here, political prisoner, which is serving a sentence in the country’s most severe prisons, writes the number one best-seller book in the recent years in his own country.”
Published by Fulton Books, Ilkin Rustemzadeh’s book recounts the moments leading up to his arrest and how, through trumped-up charges and a past record of political activism, he was targeted for speaking out against the human rights violations of his country. After being pardoned, Rustemzadeh shares his story in the hopes of raising awareness of what wrongs many endure in a country many have not heard of before.
Readers who wish to experience this chilling work can purchase “Tale of Darkness” online at the Apple iTunes Store, Amazon, Google Play, or Barnes and Noble.
Please direct all media inquiries to Author Support via email at [email protected] or via telephone at 877-210-0816.
Contact Information:
Media Relations
[email protected]
Press Release Service
by
Newswire.com
Money Saved, Trees Embraced: How Tenere is Accelerating Global Reforestation Efforts
 Designed to Save Consumers Money While Giving Back to the Environment, Tenere is on Target to Plant Six Million Trees by 2024
Designed to Save Consumers Money While Giving Back to the Environment, Tenere is on Target to Plant Six Million Trees by 2024
TREES Certificate of …

