The Global Healthcare Leaders Foundation (GHLF) Stands to Revolutionize the Industry Through an Unwavering Focus on Healthcare Excellence to Ignite Leadership and Innovation
 BOSTON, May 8, 2024 (Newswire.com)
BOSTON, May 8, 2024 (Newswire.com)
–
The Global Healthcare Leaders Foundation (GHLF) is proud to announce its official launch, representing a new coalit…
Lifescapes Counseling Offers ‘Is It Time for Medication?’ Webinar
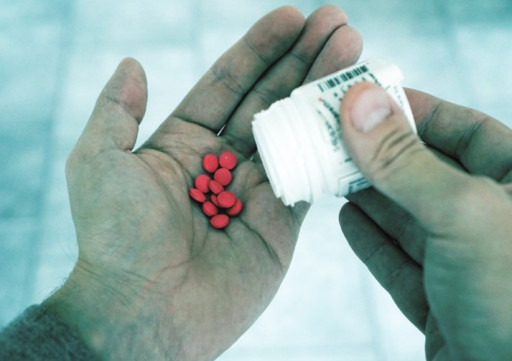 Connecting People to Professionals
Connecting People to Professionals
APEX, N.C., May 8, 2024 (Newswire.com)
–
Wondering if it is time to start medication for a mental health issue? Do you wish you could get an honest answer to your questions about psychotropic medication without committing to treatment or waiting forever for an appointment? What if you could anonymously ask a panel of legitimate professionals your most private questions in real time?
Well, that opportunity is here. A local mental health practice will soon give the public a chance to ask their burning psychiatric medication questions. On May 16th, Lifescapes Counseling will present their second in a series of educational webinars, live via video in an interactive, question-and-answer format. “Is It Time for Medication?” will be presented by experienced licensed mental health providers Kathryn Wriggelsworth, DNP, PMHNP-BC, Ram Miro, DNP, PMHNP-BC, and Amy Moulds, MEd, LCMHCS.
During this webinar, we’ll present basic information about psychotropic medication, including risks/benefits/side effects. We will also explore the reasons many people are anxious to go the psychiatry route and how to decide if it is time to try meds. Participants will also learn why such referrals are made, and how to find a medication provider with whom they feel comfortable.
The ACCESSlifescapes Webinar Series uses a large group environment to reach people with questions. “We want to provide the opportunity to chat with real therapists in real time at low cost,” says Practice Director Amy Moulds. “It can be hard to identify trustworthy information online, and many people would be surprised to know how common their most guarded questions actually are.”
As therapy requires financial and scheduling commitments that are not feasible for everyone, ACCESSlifescapes offers the opportunity to interact directly with licensed professionals about a range of timely psychological, psychiatric, and behavioral issues, such as school refusal, psychiatric medication, parenting LGBTQIA+ adolescents, eating disorders, etc. This webinar format will allow participants (who will not be visible) to receive peer support and validation in a private setting.
Participants will have the opportunity to submit questions privately when completing the online registration. This allows the presenting clinicians to organize the webinar in a manner that will prioritize the most requested information. Participants can ask their questions anonymously during the webinar. Registration is limited and can be accessed here: https://us06web.zoom.us/webinar/register/WN_uWvbppPGRE-QrZf82PCmug.
Past webinar recordings are also available at our website, including “Dealing Effectively with School Refusal.” If you would like to register for the “Is It Time for Medication?” webinar, or to access the schedule of future webinar topics, please visit www.lifescapescounseling.com/accesslifescapes-webinars.
For questions about the ACCESSlifescapes Webinar Series, please call us at 919.303.0273 or email us at [email protected].
Lifescapes Counseling Associates, PLLC is an outpatient mental health practice providing professional psychiatric, psychological, and therapeutic services. Psychological evaluations, medication management, individual, couples, group, and family counseling services are available. Find us at Bradley Commons – 950 Windy Rd, Suite 305, Apex NC 27502 or at www.lifescapescounseling.com, or 919.303.0273.
Contact Information:
Amy Moulds
Practice Director
9193030273
Original Source:
Lifescapes Counseling Offers ‘Is It Time for Medication?’ Webinar
Throtle and StackAdapt Collaboration Accelerates Healthcare Audience Targeting Through Advanced Onboarding and Activation Solutions
 Throtle and Stackadapt
Throtle and Stackadapt
RED BANK, N.J., May 8, 2024 (Newswire.com)
–
Throtle, healthcare’s only dedicated identity resolution provider, and StackAdapt, …
KidsEmail.org Acquired by Internet Safety Expert JT Smith in Strategic Move to Enhance Child Digital Safety

Kids Email Logo
ROCKVILLE, Utah & ARBON, Idaho, May 8, 2024 (Newswire.com)
–
Jacob Andersen, the founder of KidsEmail.org, a leading child safety platf…
The post KidsEmail.org Acquired by Internet Safety Expert JT Smith in Strategic Move to Enhance Child Digital Safety first appeared on RSVTV news.
Freyja HealthCare’s VereSee™ Device Receives 510(k) Clearance, the First of Many Products

Freyja Healthcare Brings First-of-Its-Kind 2mm Abdominal-Access Device to Laparoscopic Surgery to Further Innovation in Fast-Growing Women’s Health Market
BOSTON, May 8, 2024 (Newswire.com)
–
Freyja Healthcare, an early-stage medical device company focused on developing a robust product portfolio of highly innovative products for women’s health, today announced that its novel VereSee device received 510(k) clearance from the United States Food and Drug Administration (FDA). Clearance from the FDA marks a critical step in enabling Freyja and VereSee — the first and only 2mm, safe abdominal-access video-entry device for laparoscopic surgery — to bring further innovation in the fast-growing women’s health market. VereSee will reinvigorate a category that has not seen innovation in over three decades.
Founded by Jón Ívar Einarsson, MD, PhD, MPH, and Gaby Moawad, MD, FACOG, Freyja’s mission is to raise the standard of women’s health in both surgical and in-office procedures, enabling physicians to deliver safe and effective care. As the company’s first novel product, the VereSee device features a proprietary design engineered to be simple for the surgeon, safe for the patient, and highly differentiated from the current standard of care. The company now has four products in advanced stages of development, 17 granted patents and 21 pending patents.
“From the beginning, I’ve been impressed with Freyja’s mission to improve gynecologic solutions for women and the providers who take care of them. With this first product, Freyja is bringing meaningful innovation to a category ready for change,” said Kim Rodriguez, longtime Freyja Executive Chairman and former CEO of Acessa Health.
There are 4.8 million laparoscopic surgeries performed annually in the United States. Abdominal entry is the most dangerous step of laparoscopic and robotic surgery in women’s health, and in all laparoscopic surgery across all specialty areas. This dangerous step results in one patient death and eight injuries in the U.S. each day[1].
“The standard of care is dangerous and outdated. Initial entry is often done blindly with Veres needles or a 5-12mm optical trocar,” said Jón Ívar Einarsson, MD, co-founder and interim CEO of Freyja. “Damaging the bowel or vasculature because of blind entry can cause significant harm, even death, if undetected.”
As a part of the FDA clearance process, Freyja performed extensive testing and documentation of the safety and efficacy of the product, validating the company’s confidence in the design of its VereSee product.
“FDA clearance has been a long process for us, and we know that this is one of the largest barriers to entry for new products in this category,” said Gaby Moawad, MD, co-founder and Board Director of Freyja. “However, we understand the importance of these devices being held to the highest standards of safety and quality. We are proud of the work our team has done to reach this milestone for Freyja.”
Freyja will soon begin initial commercialization of the VereSee device, with broad availability expected in the second half of 2024.
About Freyja Healthcare
Freyja Healthcare was founded in 2017, has four products in advanced stages of development, 17 granted patents and 21 pending patents, and is engineering a robust product portfolio focused on safe and effective surgical and in-office solutions for women. Founded by two well-respected, minimally invasive gynecologic surgeons, Jón Ívar Einarsson, MD, PhD, MPH, Past-President of AAGL, founder of the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Brigham and Women’s Hospital (BWH) and a Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School, and Gaby Moawad, MD, FACOG, Associate Professor of Obstetrics and Gynecology at The George Washington University School of Medicine and Health Sciences (GWSMHS). Drs. Einarsson and Moawad are inventing and building the products they wish they had early in their careers and elevating the standards for products used in women’s health surgical and in-office care. Freyja has raised almost $8 million in seed funding from private and professional high net-worth investors. Learn more at Freyja Healthcare.
[1] Clark NV, Einarsson JI. Laparoscopic entry techniques. In: Minimally invasive surgery in gynecologic practice. De Gruyter 2020.
Contact Information:
Jerry Brecher
Original Source:
Freyja HealthCare’s VereSee™ Device Receives 510(k) Clearance, the First of Many Products
The post Freyja HealthCare’s VereSee™ Device Receives 510(k) Clearance, the First of Many Products first appeared on RSVTV news.
Clear Cannabis, Inc. Expands ‘The Clear’ Brand in New Jersey With Kushi Labs

Announcement Coincides with Major Marijuana Reclassification Announcements from The White House Administration

Kushi Labs, Inc. Logo
Kushi Labs, Inc. Logo
DENVER, May 8, 2024 (Newswire.com)
–
In response to growing consumer demand, Clear Cannabis, Inc. (CCI), a leader in cannabis innovation and quality since 2013, is thrilled to announce the expansion of its popular brand, The CLEARTM to New Jersey through a strategic partnership with Kushi Labs. This collaboration marks a significant milestone as the company introduces its top-tier cannabis products to The Garden State. This marks the 12th state for The Clear and its market expansion.
Under this agreement, CCI will license its IP formulas, processes, and operational knowledge to Kushi Labs.
“The CLEAR has consistently set the benchmark for excellence and innovation in the cannabis market,” said Richard M. Batenburg, Jr., CEO of Clear Cannabis, Inc. “Our decision to bring The CLEAR to New Jersey with Kushi Labs is a testament to our commitment to meet consumer demand and deliver unparalleled cannabis experiences.”
The CLEAR’s products are celebrated for their unparalleled purity, potency, and palate-pleasing flavors, setting the industry standard for cannabis product excellence.
This expansion into New Jersey is driven by a strong market demand and a commitment to making these superior products more accessible to discerning consumers in more states.
Kushi Labs, known in the New Jersey cannabis sector for its exceptional manufacturing processes, will serve as the licensed partner, ensuring that the same high-quality standards known across the U.S. are upheld. The CLEAR’s entry into New Jersey is anticipated to set new benchmarks in the market for product quality and consumer satisfaction.
“Our partnership with Clear Cannabis, Inc. represents a pivotal development in our mission to offer the best cannabis products to New Jersey residents,” stated Ajay Patel, CEO of Kushi Labs. “The CLEAR’s reputation for quality and trust makes them the ideal partner for us as we look to elevate the cannabis experience in our state.”
This expansion into New Jersey coincides with the Biden administration’s recent announcement to reclassify marijuana as a class-3 drug, easing restrictions on its use and research.
“The reclassification of marijuana by the White House is a significant step towards greater acceptance and accessibility of cannabis products, and The CLEAR is proud to be at the forefront of this movement,” said Batenburg.
About The Clear Cannabis, Inc.:
Since its inception in 2013, The CLEAR (part of Clear Cannabis, Inc.) has been at the innovation forefront of the cannabis industry, offering a diverse range of products from vapes to edibles, pre-rolled joints and more. Known for its exceptional flavors and consistent quality, The CLEAR has become one of the most trusted and awarded cannabis brands in the United States.
From Mild to Wild and Everything Between, The CLEAR has something for everyone, catering to a wide array of lifestyles and preferences, ensuring a premium experience to be enjoyed by all.
For additional information about Clear Cannabis, Inc. and The CLEAR, please visit https://clearcannabisinc.com/.
About Kushi Labs:
Founded in 2021, Kushi Labs is a leading Licensed Cannabis Manufacturing facility in New Jersey. Dedicated to becoming the primary supplier for cultivators and dispensaries statewide, the company emphasizes innovation and excellence. By integrating advanced technology, Kushi Labs sets high standards in cannabis manufacturing quality and efficiency. Supported by a diverse team of engineers, scientists, businessmen, and connoisseurs, all united by their passion, Kushi Labs commits to delivering outstanding products and services as an industry leader.
Contact Information:
Brett Schklar
CMO
3038832449
Related Images

Kushi Labs, Inc. Logo
Kushi Labs, Inc. Logo

The CLEAR Logo
The CLEAR Logo
Original Source:
Clear Cannabis, Inc. Expands ‘The Clear’ Brand in New Jersey With Kushi Labs
The post Clear Cannabis, Inc. Expands ‘The Clear’ Brand in New Jersey With Kushi Labs first appeared on RSVTV news.
Tiger Aesthetics Medical, LLC Acquires Assets of Sientra, Inc.
Focus on Regenerative Medicine Combined with Best-in-Class Aesthetics Device Offering
FRANKLIN, Wis., April 24, 2024 (New…
Essense of Australia Celebrates ‘That First Look’ in New Collection

Timeless bridal gowns for a memorable wedding day

M24 ESS D4123
M24 ESS D4123
LENEXA, Kan., April 24, 2024 (Newswire.com)
–
Classic yet modern bridal style takes center stage in the newest collection from globally renowned wedding dress designer Essense of Australia. Designed for every bridal vision and love story, the new collection delivers beautiful wedding gowns for a dream-worthy wedding day.
“Brides are dreaming of celebrating their next chapter in a stunning wedding dress with the most unforgettable details,” says Martine Harris, Chief Creative Officer, Essense of Australia. “The latest collection offers eye-catching wedding gowns that evoke ‘that first look’ kind of feeling.”
Effortless beauty comes to life in the newest collection. Elevated necklines, like square, sweetheart and halters, let brides make a statement on and off the aisle. Strapless and off-the-shoulder silhouettes offer a chic update to traditional bridal styles, while detachable accessories, like long sleeves and bows, add a touch of elegance and glamour. Romantic design details, from intricate beading and gorgeous pearls to 3D floral lace and hints of sparkle and shine, add head-turning style to any bridal silhouette. A striking new color, French Blue, gives brides a chance to showcase their captivating aisle style.
With so many breathtaking gowns to choose from, brides can find a dress that lets them celebrate their most special moment on and off the aisle. The new Essense of Australia collection is now available at a retailer near you, featuring over 27 new gowns. Gowns are available in U.S. sizes 2 to 20, with many styles available in the EveryBody/EveryBride collection for U.S. sizes 22 to 34. To view the entire collection or find a store, visit www.essensedesigns.com.
About Essense of Australia
Essense of Australia is a leading international bridal design house and wholesaler that creates and manufactures award-winning gowns for independent bridal retailers throughout the world under labels Stella York, Essense of Australia, Martina Liana, Martina Liana Luxe and All Who Wander, as well as private label collection Oxford Street and bridesmaid label Sorella Vita. The Essense of Australia family of brands can be found at more than 1,200 retailers worldwide including the U.S., Canada, U.K., Australia, New Zealand and throughout Europe.
Contact Information:
Lindsay Santee
Associate Director of Strategic Communications
9139098623
Original Source:
Essense of Australia Celebrates ‘That First Look’ in New Collection
The post Essense of Australia Celebrates ‘That First Look’ in New Collection first appeared on RSVTV news.
EarthCruiser Announces Closure After 16 Years of Overlanding Innovation

Company to Discontinue Production of Vehicles and Service
EarthCruiser EarthCruiser
BEND, Ore., April 24, 2024 (N…
The post EarthCruiser Announces Closure After 16 Years of Overlanding Innovation first appeared on RSVTV news.
Assured Imaging Expands Into Virginia Through Pivotal Partnership, Advancing Its Commitment to Saving Lives Through Innovation in Underserved Areas
 Assured Imaging Assured logo
Assured Imaging Assured logo
LEESBURG, Va., April 24, 2024 (Newswire.com)
–
A partnership with HealthWorks for Northern Virginia brings Rezolut, the p…
