Design Veronique Introduces the First Post Surgical Compression Garment With Back Zipper
 Mommy Makeover Medical “Compression Garment Therapy™” Recovery System
Mommy Makeover Medical “Compression Garment Therapy™” Recovery System
Mommy Make Over Compression Garment POST SURGICAL COMPRESSION GARMENT WITH …
NuLoupes Receives FDA Approval, Setting a New Standard in Dental and Surgical Visualization
 NEWPORT BEACH, Calif., December 15, 2023 (Newswire.com)
NEWPORT BEACH, Calif., December 15, 2023 (Newswire.com)
–
NuEyes, a pioneer in the field of augmented reality smart glasses technology, is proud to anno…
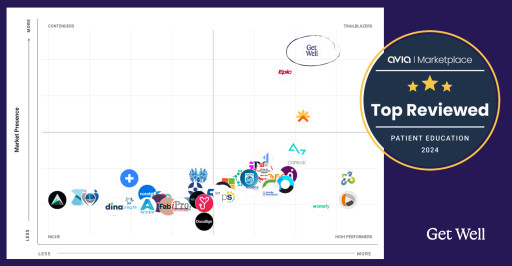
Get Well Named to AVIA Marketplace’s Top Patient Education Companies
BETHESDA, Md., December 14, 2023 (Newswire.com)
–
Get Well, the global leader in consumer digital patient engagement, announced today that it was named a Top Reviewed Company in Patient Education, following thorough research and industry outcomes by AVIA Marketplace, the premier digital health marketplace.
The Top Patient Education Companies Report is a synthesis and examination of client ratings, reviews, and healthcare system implementation data from all over the country. The report highlights industry trends and underscores the profound impact companies and their products are making in the field. Given the rapid advancements and the immense challenges in care, this report elucidates the landscape and how these innovative solutions best aid organizations to revolutionize their patient care.
The Get Well 360 platform is designed to activate and engage patients in the community, at the point of care and beyond. Their commitment to providing patients and families the right information at the right time has long established them as the leader in the space. With a large library of clinically validated digital care plans, healthcare organizations can use Get Well to guide patients through pre-admission procedures and surgery prep, deliver education during inpatient stays, and provide ongoing touchpoints through virtual check-ins and two-way communication.
“We are thrilled and honored to be recognized as a Top Patient Education Company by AVIA Marketplace. Get Well has long been a leader in patient engagement and digital education and we’ve just invested three years and over $50 million dollars in developing a best-in-class consumer digital platform that will continue to drive meaningful results to healthcare organizations and the communities they serve,” said Michael O’Neil, Founder and Chief Executive Officer of Get Well. “This distinction further validates that our hard work and investments are paying off.”
For over 20 years, Get Well has provided digital patient engagement across more than 600 provider clients, reaching over eight million patients annually across all care settings. Their pre-admission and post-discharge digital care plans averaged 95,000 interactions and over 130,000 interactions per month. The company’s solutions are live in over 75,000 hospital beds and more than 230 clinics.
About Get Well
Get Well empowers the communities you serve to take control of their healthcare journey. With the Get Well 360 Platform, healthcare organizations can now deliver consumer-centered experiences that build lifelong loyalty while deepening patient relationships, improving clinical quality, and growing market share. Each year, we empower organizations to activate, engage, and retain millions of patients. Learn more at getwellnetwork.com or follow Get Well on LinkedIn and Twitter.
About AVIA
AVIA is the nation’s leading digital transformation partner for healthcare organizations. AVIA provides unique market intelligence, proven collaborative tools, and results-based consulting to help solve healthcare’s biggest strategic challenges. Learn more about AVIA and AVIA Marketplace, the industry’s premier marketplace for digital health innovation, at aviahealthinnovation.com.
Contact Information:
Rachel Allen
Director, Corporate Marketing & Communications
(480) 993-7149
Original Source:
Get Well Named to AVIA Marketplace’s Top Patient Education Companies
CLS Health Expands Reach With New Primary Care Facility in Pearland
 CLS Health, a prominent physician-owned healthcare medical group, is excited to announce the opening of its latest primary care facility, CLS Health Shadow Creek Primary Care Clinic, located at 10905 Memorial Hermann D…
CLS Health, a prominent physician-owned healthcare medical group, is excited to announce the opening of its latest primary care facility, CLS Health Shadow Creek Primary Care Clinic, located at 10905 Memorial Hermann D…
Omni-Biotic AB 10 Probiotic Wins Practitioner’s Choice Award
 Omni-Biotic AB 10 with Sachet Omni-Biotic AB 10 with Sachet
Omni-Biotic AB 10 with Sachet Omni-Biotic AB 10 with Sachet
NEW YORK, December 13, 2023 (Newswire.com)
–
AllergoSan USA is thrilled to announce that t…
ASTCT Response to FDA Approval of Gene Therapies for Treatment of Sickle Cell Disease (SCD)
 ASTCT logo The American Society for Transplantation and Cellular Therapy is an international professional membership association.
ASTCT logo The American Society for Transplantation and Cellular Therapy is an international professional membership association.
CHICAGO, December 13, 2023 (News…
Research for the Treatment of a ‘Black Sheep’ Cancer Moves to Human Clinical Trials
With $3.5 million in funding from the GI Research Foundation, a team at the MD Anderson Cancer Center explores a one-two punch to combat BRAF-mutated colorectal cancer and its “dismal prognosis.”
CHICAGO, December 13, 2023 (Newswire.com)
–
Many cancers caused by the mutation of the BRAF gene respond well to a class of immunotherapies known as BRAF-inhibitors. BRAF-mutated colorectal cancer, however, almost never does. Doctors have known this for years, but they are yet to find a treatment that works well for most patients.
“BRAF-inhibitors provide very different clinical outcomes,” said Dr. Scott Kopetz, a Professor and Associate Vice President for Translational Integration at The University of Texas MD Anderson Cancer Center. “They work amazingly well in melanoma, for example, but are less effective in colorectal cancer.”
“BRAF [in colorectal cancer] is that odd uncle who doesn’t behave like the rest of the family. The black sheep. Is he even related? The usual rules don’t apply.”
Dr. Kopetz and his team’s most recent studies of this black sheep are now moving from research in mice to clinical trials in humans. These trials were approved by the National Cancer Institute in October and will launch in early 2024.
The GI Research Foundation helped support the work with mice models, along with several other aspects of the team’s BRAF-related research. The $3.5 million grant is part of the foundation’s massive CA CURE initiative.
“Last year, we were able to deliver more than $18 million to researchers across the country to improve diagnostics and develop immunotherapies and personalized vaccines in gastrointestinal cancers through CA CURE,” said Katie Chudnovsky, chair of the GI Research Foundation’s Board of Directors. “Patients need treatments like those being developed by Dr. Kopetz – so very urgently – and we are thrilled to see this team’s work moving to clinical trials so quickly.”
BRAF-mutated colorectal cancer comes with a “dismal prognosis,” according to research in the International Journal of Molecular Sciences, and the median patient survives less than a year.
This new form of treatment for BRAF-mutated colorectal cancer relies on what Kopetz called a “two-hit hypothesis.” That is, these cancers are caused by the combination of mutations in the BRAF gene and epigenetic dysregulation. Epigenetic dysregulation refers to the fact that behaviors and environment can impact how a person’s genes behave.
Here, researchers have found that a mutation to the BRAF gene changes the production of proteins that cause healthy cells to grow into cancerous tumors. Other forms of BRAF-mutated cancer respond well to BRAF-inhibitor immunotherapy that can halt the production of those proteins.
In BRAF-mutated colorectal cancer, however, another piece of DNA is unable to express itself entirely. Those genes typically create natural tumor suppressors that fight early cancer growth. Carbon molecules called methyl groups attach themselves to the DNA and prevent the DNA from building cancer-suppressing proteins. That “methylation” is the epigenetic dysregulation in this case, likely caused by inflammation, diet, or external environmental factors.
If doctors can stop the proteins that cause cancer and stop the bromodomains, they might be able to produce a one-two punch to combat their double hit hypothesis. The treatment would slow BRAF-mutated colorectal cancer growth and encourage natural cancer suppressors.
That idea will be tested in the human trials that were recently approved by the National Cancer Institute. A BRAF-inhibitor, an EGFR-inhibitor, and a bromodomain-inhibitor will be administered to patients. This combined treatment was developed by Kopetz and his colleague Dr. Kunal Rai, an Associate Professor of Genomic Medicine at MD Anderson.
The research moved from the GI Research Foundation-funded mouse models to human trials exceptionally quickly, according to Kopetz.
“It’s rare for the pieces to come together like this,” he said. “Lots of times, this would be two years of work to get the human trials up and running. But we were able to show how clearly this approach worked in multiple mouse models and that really sped things up.”
Other funding from the GI Research Foundation will help speed things up even further. “The new technologies and techniques that Dr. Kopetz and his team are exploring will give them the ability to innovate that much faster. It provides the entire community with the means to gather high-quality data more quickly and get it into trials and ultimately into the clinic,” said Dr. David Rubin. Rubin is the Joseph B. Kirsner Professor of Medicine and Chief of the Section of Gastroenterology, Hepatology and Nutrition at University of Chicago Medicine. He is also GI Research Foundation’s Senior Scientific Advisor.
“The GI Research Foundation has a legacy of funding high risk-high reward and innovative science, and doing so efficiently and extraordinarily effectively. Kopetz and his colleagues were recipients of a dedicated campaign that we launched in 2022 to tackle the most difficult challenges of colorectal cancer.”
“Support from the GI Research Foundation allows us to accelerate our work and try some out of the box stuff,” Kopetz said. “We have amazing animal models, but there’s no substitute for understanding cancer and treatments with the patients themselves. We want to develop the tools we need so we can learn as much as possible from them and help them as much and as quickly as we can.”
About the GI Research Foundation
The GI Research Foundation was founded in 1961 by grateful patients and friends of the late Dr. Joseph B. Kirsner, a pioneer in gastroenterology who devoted his life to medicine, teaching, and patient care. Today, the University of Chicago Medicine’s Digestive Diseases Center, which is supported by the foundation, is internationally recognized for research-driven medicine and its team of highly specialized physicians. For more information, see: https://giresearchfoundation.org/
About the University of Chicago Digestive Diseases Center
The Digestive Diseases Center at the University of Chicago Medicine is a collaborative, multidisciplinary network of physicians, researchers and affiliated health professionals who share a legacy of innovation and a common purpose: to improve the lives of patients who suffer from digestive diseases. For more information, see: https://www.uchicagomedicine.org/conditions-services/digestive-diseases
Contact Information:
Jackie Casey
Executive Director
312-332-1350
Original Source:
Research for the Treatment of a ‘Black Sheep’ Cancer Moves to Human Clinical Trials
Research for the Treatment of a ‘Black Sheep’ Cancer Moves to Human Clinical Trials
With $3.5 million in funding from the GI Research Foundation, a team at the MD Anderson Cancer Center explores a one-two punch to combat BRAF-mutated colorectal cancer and its “dismal prognosis.”
CHICAGO, December 13, 2023 (Newswire.com)
–
Many cancers caused by the mutation of the BRAF gene respond well to a class of immunotherapies known as BRAF-inhibitors. BRAF-mutated colorectal cancer, however, almost never does. Doctors have known this for years, but they are yet to find a treatment that works well for most patients.
“BRAF-inhibitors provide very different clinical outcomes,” said Dr. Scott Kopetz, a Professor and Associate Vice President for Translational Integration at The University of Texas MD Anderson Cancer Center. “They work amazingly well in melanoma, for example, but are less effective in colorectal cancer.”
“BRAF [in colorectal cancer] is that odd uncle who doesn’t behave like the rest of the family. The black sheep. Is he even related? The usual rules don’t apply.”
Dr. Kopetz and his team’s most recent studies of this black sheep are now moving from research in mice to clinical trials in humans. These trials were approved by the National Cancer Institute in October and will launch in early 2024.
The GI Research Foundation helped support the work with mice models, along with several other aspects of the team’s BRAF-related research. The $3.5 million grant is part of the foundation’s massive CA CURE initiative.
“Last year, we were able to deliver more than $18 million to researchers across the country to improve diagnostics and develop immunotherapies and personalized vaccines in gastrointestinal cancers through CA CURE,” said Katie Chudnovsky, chair of the GI Research Foundation’s Board of Directors. “Patients need treatments like those being developed by Dr. Kopetz – so very urgently – and we are thrilled to see this team’s work moving to clinical trials so quickly.”
BRAF-mutated colorectal cancer comes with a “dismal prognosis,” according to research in the International Journal of Molecular Sciences, and the median patient survives less than a year.
This new form of treatment for BRAF-mutated colorectal cancer relies on what Kopetz called a “two-hit hypothesis.” That is, these cancers are caused by the combination of mutations in the BRAF gene and epigenetic dysregulation. Epigenetic dysregulation refers to the fact that behaviors and environment can impact how a person’s genes behave.
Here, researchers have found that a mutation to the BRAF gene changes the production of proteins that cause healthy cells to grow into cancerous tumors. Other forms of BRAF-mutated cancer respond well to BRAF-inhibitor immunotherapy that can halt the production of those proteins.
In BRAF-mutated colorectal cancer, however, another piece of DNA is unable to express itself entirely. Those genes typically create natural tumor suppressors that fight early cancer growth. Carbon molecules called methyl groups attach themselves to the DNA and prevent the DNA from building cancer-suppressing proteins. That “methylation” is the epigenetic dysregulation in this case, likely caused by inflammation, diet, or external environmental factors.
If doctors can stop the proteins that cause cancer and stop the bromodomains, they might be able to produce a one-two punch to combat their double hit hypothesis. The treatment would slow BRAF-mutated colorectal cancer growth and encourage natural cancer suppressors.
That idea will be tested in the human trials that were recently approved by the National Cancer Institute. A BRAF-inhibitor, an EGFR-inhibitor, and a bromodomain-inhibitor will be administered to patients. This combined treatment was developed by Kopetz and his colleague Dr. Kunal Rai, an Associate Professor of Genomic Medicine at MD Anderson.
The research moved from the GI Research Foundation-funded mouse models to human trials exceptionally quickly, according to Kopetz.
“It’s rare for the pieces to come together like this,” he said. “Lots of times, this would be two years of work to get the human trials up and running. But we were able to show how clearly this approach worked in multiple mouse models and that really sped things up.”
Other funding from the GI Research Foundation will help speed things up even further. “The new technologies and techniques that Dr. Kopetz and his team are exploring will give them the ability to innovate that much faster. It provides the entire community with the means to gather high-quality data more quickly and get it into trials and ultimately into the clinic,” said Dr. David Rubin. Rubin is the Joseph B. Kirsner Professor of Medicine and Chief of the Section of Gastroenterology, Hepatology and Nutrition at University of Chicago Medicine. He is also GI Research Foundation’s Senior Scientific Advisor.
“The GI Research Foundation has a legacy of funding high risk-high reward and innovative science, and doing so efficiently and extraordinarily effectively. Kopetz and his colleagues were recipients of a dedicated campaign that we launched in 2022 to tackle the most difficult challenges of colorectal cancer.”
“Support from the GI Research Foundation allows us to accelerate our work and try some out of the box stuff,” Kopetz said. “We have amazing animal models, but there’s no substitute for understanding cancer and treatments with the patients themselves. We want to develop the tools we need so we can learn as much as possible from them and help them as much and as quickly as we can.”
About the GI Research Foundation
The GI Research Foundation was founded in 1961 by grateful patients and friends of the late Dr. Joseph B. Kirsner, a pioneer in gastroenterology who devoted his life to medicine, teaching, and patient care. Today, the University of Chicago Medicine’s Digestive Diseases Center, which is supported by the foundation, is internationally recognized for research-driven medicine and its team of highly specialized physicians. For more information, see: https://giresearchfoundation.org/
About the University of Chicago Digestive Diseases Center
The Digestive Diseases Center at the University of Chicago Medicine is a collaborative, multidisciplinary network of physicians, researchers and affiliated health professionals who share a legacy of innovation and a common purpose: to improve the lives of patients who suffer from digestive diseases. For more information, see: https://www.uchicagomedicine.org/conditions-services/digestive-diseases
Contact Information:
Jackie Casey
Executive Director
312-332-1350
Original Source:
Research for the Treatment of a ‘Black Sheep’ Cancer Moves to Human Clinical Trials
TridentCare to Grow and Enhance Its Laboratory Services Business With the Addition of a Seasoned Diagnostic Operator
 TridentCare
TridentCare
SPARKS, Md., December 12, 2023 (Newswire.com)
–
TridentCare is pleased to announce the addition of Chris Tomlinson, MBA, CRA, FAHRA to the …
Preventogen is a New Tool in the Fight Against Antibiotic-Resistant Infections Caused by Superbugs, a Leading Cause of Death Worldwide
 Preventogen’s FDA-Cleared Microbicidal Liquid Polymer Ensures the Eradication of These Dangerous Pathogens on Contact While Forming a More Effective and Measurable Barrier to Optimize Healing
Preventogen’s FDA-Cleared Microbicidal Liquid Polymer Ensures the Eradication of These Dangerous Pathogens on Contact While Forming a More Effective and Measurable Barrier to Optimize Healing
…
